Sarepta Stock Forecast
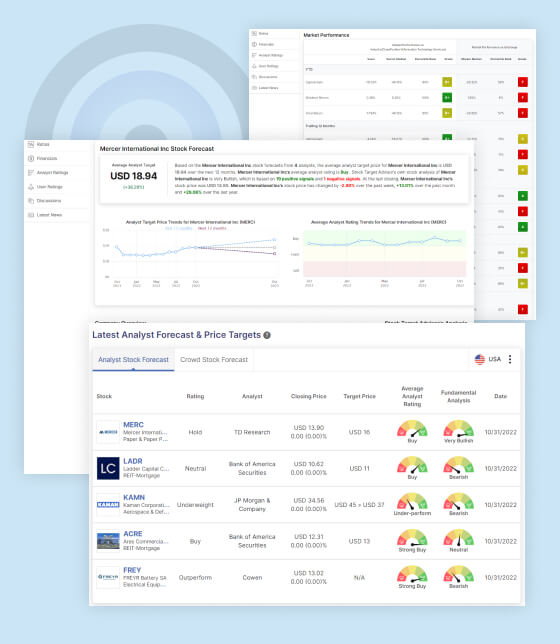
The average target price for Sarepta Therapeutics Inc (SRPT) stock, as forecasted by 14 analysts, is USD 144.23 over the next 12 months. This suggests that the average analyst has a bullish outlook on the stock and sees the potential for significant gains in the next year. On average, the analysts rate the stock as “Strong Buy”, indicating a positive outlook and potential for significant returns. Stock Target Advisor has its own analysis of SRPT, which is “Slightly Bearish”. This is based on a combination of positive and negative signals which could be a combination of technical, fundamental and quantitative analysis. Currently, the stock is trading at USD 120.13, which indicates a drop of -3.88% over the past week, +1.50% over the past month and +57.36% over the last year.
Bullish Case for Sarepta’s Stock
Sarepta Therapeutics is a biopharma company that is using gene editing approach to develop treatments for genetically-based disease conditions, with a focus on muscular dystrophy. They have a comprehensive product and research lineup that includes drug candidates in the discovery and clinical stages of development, as well as approved products in the commercialization stages.
On the commercial side, Sarepta has three approved gene therapy products on the market for the treatment of Duchenne muscular dystrophy. These three drugs, Exondys 51, Vyondys 53, and Amondys 45, brought in a total of $208 million in product revenues for third quarter lastcyear., which was up 24 percent year over year, and added a significant amount to the $230 in revenue for the company. They also recently released preliminary fourth quarter and full-year product revenue results that showed net product revenues for the quarter are expected to be $235.5 million, 34 percent increase. Revenues for the year are expected to reach $843. million, which is above guidance of $825 to $840 million.
The most important program in the pipeline is SRP-9001 that Sarepta is developing with Roche. It is a potential treatment for ambulant patients with Duchenne and based on positive clinical trial results, the company submitted the Biologics License Application to the FDA in September and is seeking accelerated approval. A PDUFA date has been set for May 29th. The company is also running the EMBARK clinical trial which is a global, randomized, double-blind, placebo-controlled study of SRP-9001, that is fully enrolled and dosed, and has proposed this trial as a confirmatory study to support accelerated approval.
UBS analyst Colin Bristow, who covers Sarepta, sees potential regulatory and clinical trial catalysts in the company’s late-stage research pipeline. He thinks that SRP-9001 has a high likelihood of accelerated approval by the FDA by May 29th and that the company has sufficient data to support the use of shortened/truncated dystrophin as a surrogate biomarker of function. He maintains a buy rating on the stock, and sets his target at $158 which implies a gain of 30 percent.
About Sarepta
Sarepta Therapeutics is a biopharmaceutical company that develops and commercializes therapies for the treatment of genetic diseases, with a focus on Duchenne muscular dystrophy (DMD). DMD is a progressive muscle disorder caused by a genetic mutation that leads to the lack of dystrophin, a protein necessary for muscle function. Sarepta’s gene editing approach aims to treat the underlying genetic cause of DMD.
The company’s flagship product is Exondys 51, a treatment for DMD that was the first FDA-approved drug to target the genetic cause of the disease. Sarepta also has two other approved DMD gene therapy products, Vyondys 53 and Amondys 45, which were approved in 2020 and 2021 respectively. These drugs are designed to address specific mutations that cause DMD.
In addition to its commercialized products, Sarepta has a robust pipeline of drug candidates in various stages of development. The company’s most advanced pipeline product is SRP-9001, which is being developed in collaboration with Roche. SRP-9001 is a potential treatment for ambulatory patients with DMD and has been submitted for FDA approval. Sarepta is also conducting a global clinical trial for SRP-9001.
Sarepta also has a research and development pipeline focused on gene therapies for other genetic diseases such as Limb-girdle muscular dystrophy (LGMD), Becker muscular dystrophy, and Myotonic dystrophy type 1 and type 2, among others. These diseases are also caused by genetic mutations that lead to the lack of specific proteins necessary for muscle function. The company’s gene editing approach aims to target these genetic causes to develop treatments for these diseases.

STA Research (StockTargetAdvisor.com) is a independent Investment Research company that specializes in stock forecasting and analysis with integrated AI, based on our platform stocktargetadvisor.com, EST 2007.